Pomaloz Cap- Pomalidomide Cap
Pomalidomide Cap
Strength: 1, 2, 4mg
Pack Size: 1 x 21
Drug Class: Antineoplastics, Angiogenesis Inhibitor
Dosage and Administration:
Pregnancy Testing Prior To Administration
Females of reproductive potential must have negative pregnancy testing and use contraception methods before initiating Pomaloz Cap.
Recommended Dosage For Multiple Myeloma
The recommended dosage of Pomaloz Cap is 1/2 mg once daily orally with or without food on Days 1 through 21 of each 28-day cycle until disease progression. Give Pomaloz Capin combination with dexamethasone.
Recommended Dosage For Kaposi Sarcoma
The recommended dosage of Pomaloz Capis 1/2 mg once daily taken orally with or without food on Days 1 through 21 of each 28-day cycle until disease progression or unacceptable toxicity. Continue HAART as HIV treatment in patients with AIDS-related Kaposi sarcoma
Cold Storage: yes
Pomaloz Cap is an immunomodulatory antineoplastic agent. The chemical name is (RS)-4-Amino-2-(2,6-dioxopiperidin-3-yl)-isoindoline-1,3-dione and it has the following chemical structure:
 |
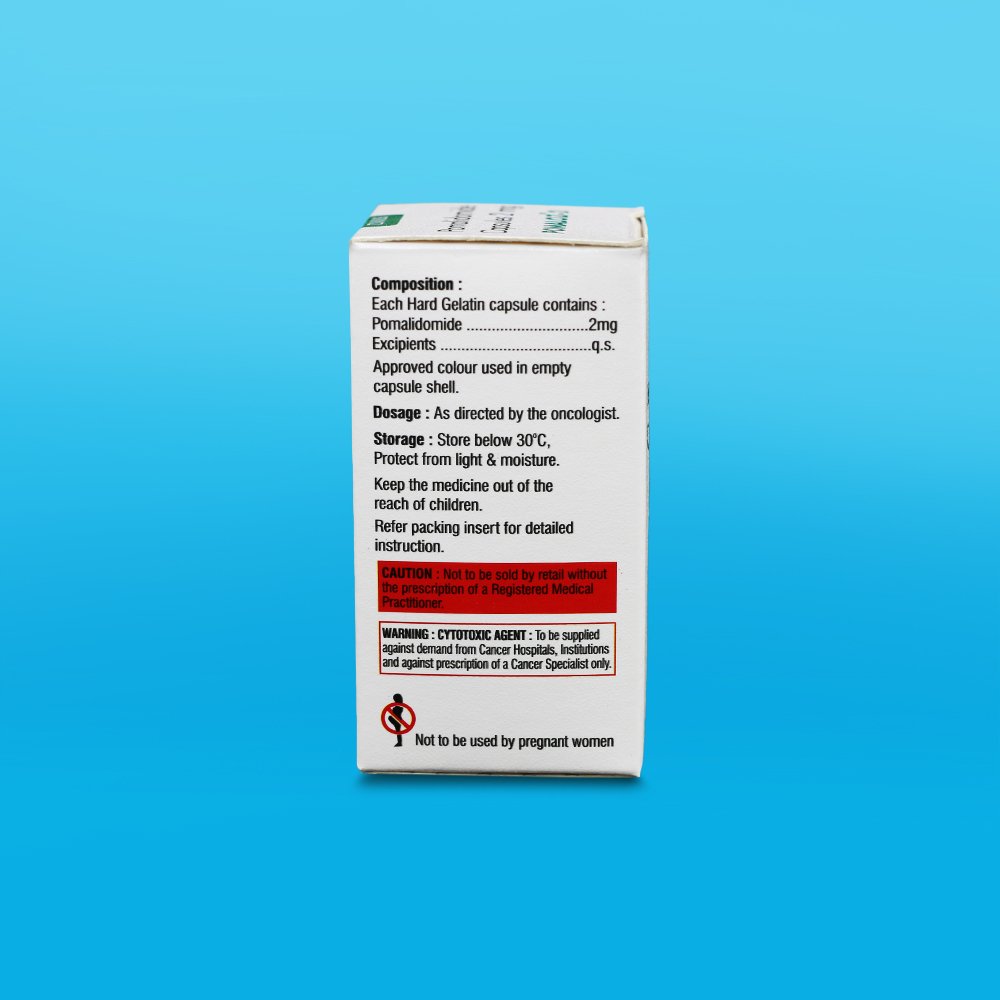
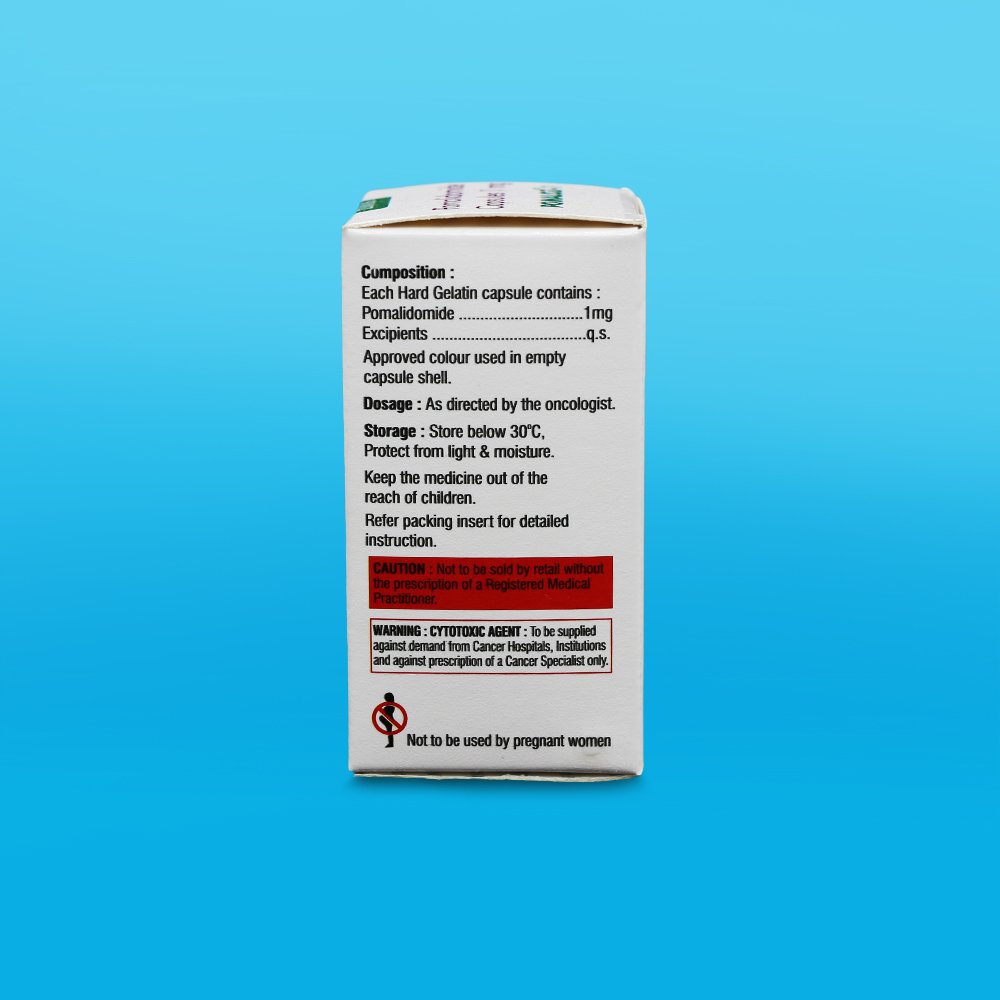
The empirical formula for pomalidomide is C13H11N3O4 and the gram molecular weight is 273.24.
Multiple Myeloma
Pomaloz Cap, in combination with dexamethasone, is indicated for adult patients with multiple myeloma (MM) who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on or within 60 days of completion of the last therapy.
Kaposi Sarcoma
Pomaloz Capis indicated for the treatment of:
- Adult patients with AIDS-related Kaposi sarcoma (KS) after failure of highly active antiretroviral therapy (HAART).
- Kaposi sarcoma (KS) in adult patients who are HIV-negative.
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Pomalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Cellular activities of pomalidomide are mediated through its target cereblon, a component of a cullin ring E3 ubiquitin ligase enzyme complex. In vitro, in the presence of drug, substrate proteins (including Aiolos and Ikaros) are targeted for ubiquitination and subsequent degradation leading to direct cytotoxic and immunomodulatory effects. Pomalidomide enhanced T cell- and natural killer (NK) cell-mediated immunity and inhibited production of pro-inflammatory cytokines (e.g., TNF-α and IL-6) by monocytes.
- Read the label carefully before use.
- Keep out of the reach of children
- Do not exceed the recommended dose
Embryo-Fetal Toxicity
Pomaloz is a thalidomide analogue and is contraindicated for use during pregnancy. Thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death.
Females Of Reproductive Potential
Females of reproductive potential must avoid pregnancy for at least 4 weeks before beginning Pomaloz therapy, during therapy, during dose interruptions and for at least 4 weeks after completing therapy.
Females Of Reproductive Potential
Females of reproductive potential must avoid pregnancy for at least 4 weeks before beginning Pomaloz therapy, during therapy, during dose interruptions and for at least 4 weeks after completing therapy.