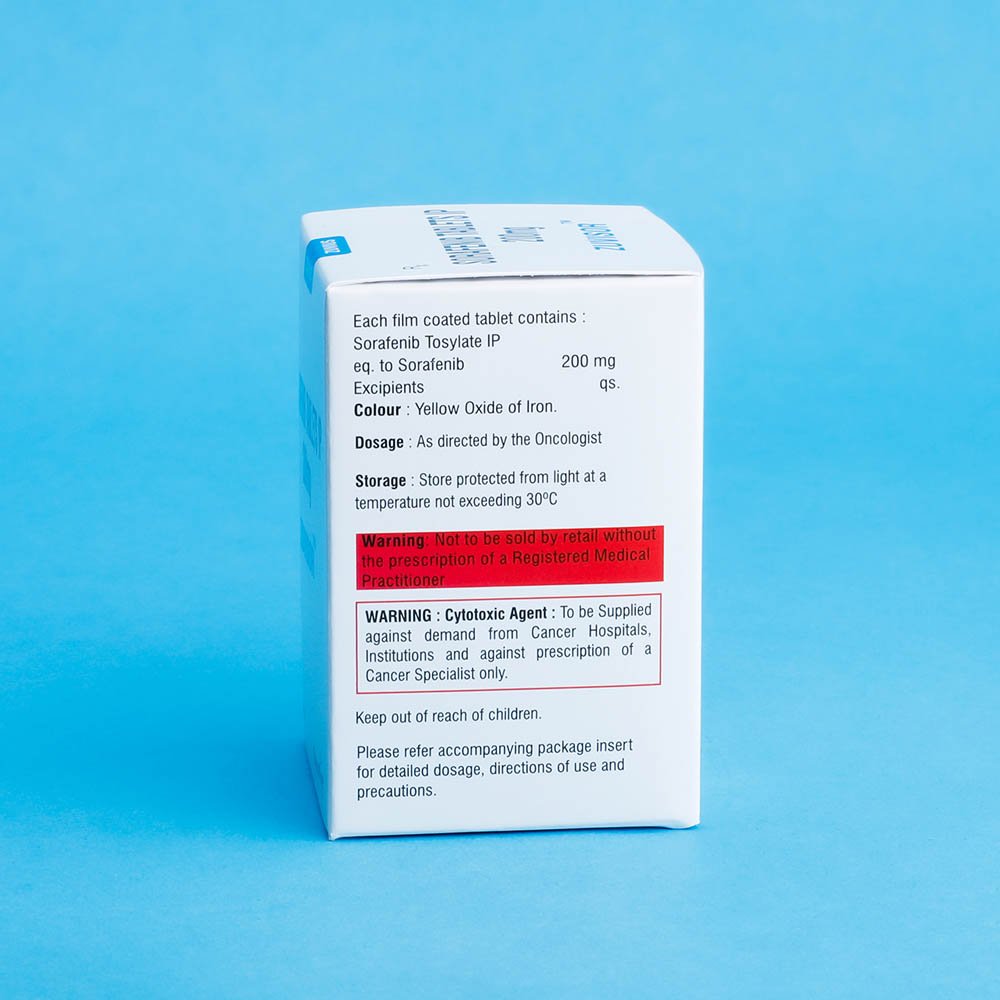
Zuvisor Tab- Sorafenib Tab 200 mg
Sorafenib Tab
Strength: 200mg
Pack Size: 1 x 30 / 1 x 120
Drug Class: Antineoplastic agents, protein kinase inhibitors
Dosage and Administration:
- The recommended dose of Sorafenib in adults is 400 mg sorafenib (two tablets of 200 mg) twice daily (equivalent to a total daily dose of 800 mg) given at least 1 hour before or 2 hours after food.
- Treatment is continued until no clinical benefit is seen or until unacceptable toxicity occurs.
- Doses are reduced to 400mg once daily if toxicity occurs; further reduction to a single dose of 400mg every other day may be necessary.
Dose adjustments
- Management of suspected adverse drug reactions may require temporary interruption or dose reduction of sorafenib therapy.
- When dose reduction is necessary during the treatment of hepatocellular carcinoma (HCC) and advanced renal cell carcinoma (RCC), Sorafenib dose should be reduced to two tablets of 200 mg sorafenib once daily (see Special Warnings and Precautions).
- When dose reduction is necessary during the treatment of differentiated thyroid carcinoma (DTC), Sorafenib dose should be reduced to 600 mg sorafenib daily in divided doses (two tablets of 200 mg and one tablet of 200 mg twelve hours apart).
- If additional dose reduction is necessary, Sorafenib may be reduced to 400 mg sorafenib daily in divided doses (two tablets of 200 mg twelve hours apart), and if necessary, further reduced to one tablet of 200 mg once daily. After the improvement of non-hematological adverse reactions, the dose of Sorafenib may be increased.
Pediatric population
The safety and efficacy of Sorafenib in children and adolescents aged < 18 years have not yet been established. No data are available.
Elderly population
No dose adjustment is required in the elderly (patients above 65 years of age).
Renal impairment
No dose adjustment is required in patients with mild, moderate, or severe renal impairment. No data is available in patients requiring dialysis. The monitoring of fluid balance and electrolytes in patients at risk of renal dysfunction is advised.
| Renal impairment | Creatinine Clearance (CC) | Recommended Starting Dose |
| Mild | Between 59 to 40 mL/min | 400mg twice daily |
| Moderate | Between 39 and 20 mL/min | 200mg twice daily |
| Severe | Less than 20 mL/min | The dose could not be defined due to low patient numbers enrolled in this cohort |
| Patients on Hemodialysis | 200mg daily | |
Hepatic impairment
Sorafenib is mainly metabolized in the liver. Hepatic impairment might reduce exposure to Sorafenib. No dose adjustment is required in patients with Child-Pugh A or B (mild to moderate) hepatic impairment. No data is available on patients with Child-Pugh C (severe) hepatic impairment.
However, a study involving patients with varying degrees of hepatic impairment recommended the following empirical starting doses of oral sorafenib, based on tolerability and the development of dose-limiting toxicity.
| Hepatic dysfunction | Bilirubin levels | Recommended Starting Dose |
| Mild | Between ULN and 1.5XULN and/or AST >ULN | 400mg twice daily |
| Moderate | Between 1.5 and 3XULN, any value of AST | 200mg twice daily |
| Severe | Over 3XULN, any value of AST | Patients unable to tolerate even 200mg every third day |
(ULN: Upper Limit of Normal)
Method of administration
For oral use
It is recommended that sorafenib should be administered without food or with a low or moderate fat meal. If the patient intends to have a high-fat meal, sorafenib tablets should be taken at least 1 hour before or 2 hours after the meal. The tablets should be swallowed with a glass of water.
Cold Storage: no
ZUVISOR is Sorafenib tablets for oral use. It is a multikinase inhibitor targeting several serine/threonine and receptor tyrosine kinases and is the tosylate salt of sorafenib.
Sorafenib tosylate has the chemical name 4-(4-{3-[4-Chloro-3-(trifluoromethyl)phenyl] ureido}phenoxy)-N2-methylpyridine-2-carboxamide 4-methyl benzenesulfonate and its structural formula is:

Sorafenib is indicated for the treatment of
- Hepatocellular carcinoma
- Advanced renal cell carcinoma who have failed prior interferon-alpha or interleukin-2 based therapy or are considered unsuitable for such therapy
- Progressive, locally advanced or metastatic, differentiated (papillary/follicular/Hürthle cell) thyroid carcinoma, refractory to radioactive iodine.
Usage-:
Sorafenib is used to treat late-stage kidney cancer (advanced renal cell carcinoma), liver cancer (hepatocellular carcinoma) that cannot be treated by surgery, and differentiated thyroid cancer that has come back or spread to other parts of your body. Sorafenib is an antineoplastic (cancer) agent.
Sorafenib is a kinase inhibitor that decreases tumor cell proliferation in vitro.
Sorafenib was shown to inhibit multiple intracellular (c-CRAF, BRAF and mutant BRAF) and cell surface kinases (KIT, FLT-3, RET, RET/PTC, VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR-ß). Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis and apoptosis. Sorafenib inhibited tumor growth of HCC, RCC, and DTC human tumor xenografts in immunocompromised mice. Reductions in tumor angiogenesis were seen in models of HCC and RCC upon sorafenib treatment, and increases in tumor apoptosis were observed in models of HCC, RCC, and DTC.
Sorafenib may cause a condition that affects the heart rhythm (QT prolongation). QT prolongation can rarely cause serious (rarely fatal) fast/irregular heartbeat and other symptoms (such as severe dizziness, fainting) that need medical attention right away.
Dermatological toxicities
- Hand foot skin reaction (palmar-plantar erythrodysaesthesia) and rash represent the most common adverse drug reactions with sorafenib. Rash and hand foot skin reaction are usually graded as CTC (Common Toxicity Criteria) Grade 1 and 2 and generally appear during the first six weeks of treatment with sorafenib.
- Management of dermatological toxicities may include topical therapies for symptomatic relief, temporary treatment interruption and/or dose modification of sorafenib, or in severe or persistent cases, permanent discontinuation of sorafenib.
Hypertension
- An increased incidence of arterial hypertension was observed in sorafenib-treated patients.
- Hypertension was usually mild to moderate, occurred early in the course of treatment, and was amenable to management with standard antihypertensive therapy.
- Blood pressure should be monitored regularly and treated, if required, in accordance with standard medical practice.
- In cases of severe or persistent hypertension, or hypertensive crisis despite institution of antihypertensive therapy, permanent discontinuation of sorafenib should be considered.
Aneurysms and artery dissections
- The use of VEGF pathway inhibitors in patients with or without hypertension may promote the formation of aneurysms and/or artery dissections.
- Before initiating Sorafenib, this risk should be carefully considered in patients with risk factors such as hypertension or history of aneurysm.
Hypoglycaemia
- Decreases in blood glucose, in some cases clinically symptomatic and requiring hospitalization due to loss of consciousness, have been reported during sorafenib treatment.
- In case of symptomatic hypoglycaemia, sorafenib should be temporarily interrupted.
- Blood glucose levels in diabetic patients should be checked regularly in order to assess if anti-diabetic medicinal product’s dosage needs to be adjusted.
Haemorrhage
An increased risk of bleeding may occur following sorafenib administration. If any bleeding event necessitates medical intervention it is recommended that permanent discontinuation of sorafenib should be considered.
Cardiac ischaemia and/or infarction
Temporary or permanent discontinuation of sorafenib should be considered in patients who develop cardiac ischaemia and/or infarction.
QT interval prolongation
Sorafenib has been shown to prolong the QT/QTc interval, which may lead to an increased risk for ventricular arrhythmias. Use sorafenib with caution in patients who have, or may develop prolongation of QTc, such as patients with a congenital long QT syndrome, patients treated with a high cumulative dose of anthracycline therapy, patients taking certain anti-arrhythmic medicines or other medicinal products that lead to QT prolongation, and those with electrolyte disturbances such as hypokalaemia, hypocalcaemia, or hypomagnesaemia.
When using sorafenib in these patients, periodic monitoring with on-treatment electrocardiograms and electrolytes (magnesium, potassium and calcium) should be considered.
Gastrointestinal perforation
Gastrointestinal perforation is an uncommon event and has been reported in less than 1% of patients taking sorafenib. In some cases, this was not associated with apparent intra-abdominal tumour. Sorafenib therapy should be discontinued.
Hepatic impairment
- No data is available on patients with Child Pugh C (severe) hepatic impairment. Since sorafenib is mainly eliminated via the hepatic route exposure might be increased in patients with severe hepatic impairment.
Warfarin co-administration
- Infrequent bleeding events or elevations in the International Normalised Ratio (INR) have been reported in some patients taking warfarin while on sorafenib therapy.
- Patients taking concomitant warfarin or phenprocoumon should be monitored regularly for changes in prothrombin time, INR or clinical bleeding episodes.
Wound healing complications
- No formal studies on the effect of sorafenib on wound healing have been conducted.
- Temporary interruption of sorafenib therapy is recommended for precautionary reasons in patients undergoing major surgical procedures.
- There is limited clinical experience regarding the timing of re-initiation of therapy following major surgical intervention. Therefore, the decision to resume sorafenib therapy following a major surgical intervention should be based on clinical judgement of adequate wound healing.
Elderly population
Cases of renal failure have been reported. Monitoring of renal function should be considered.