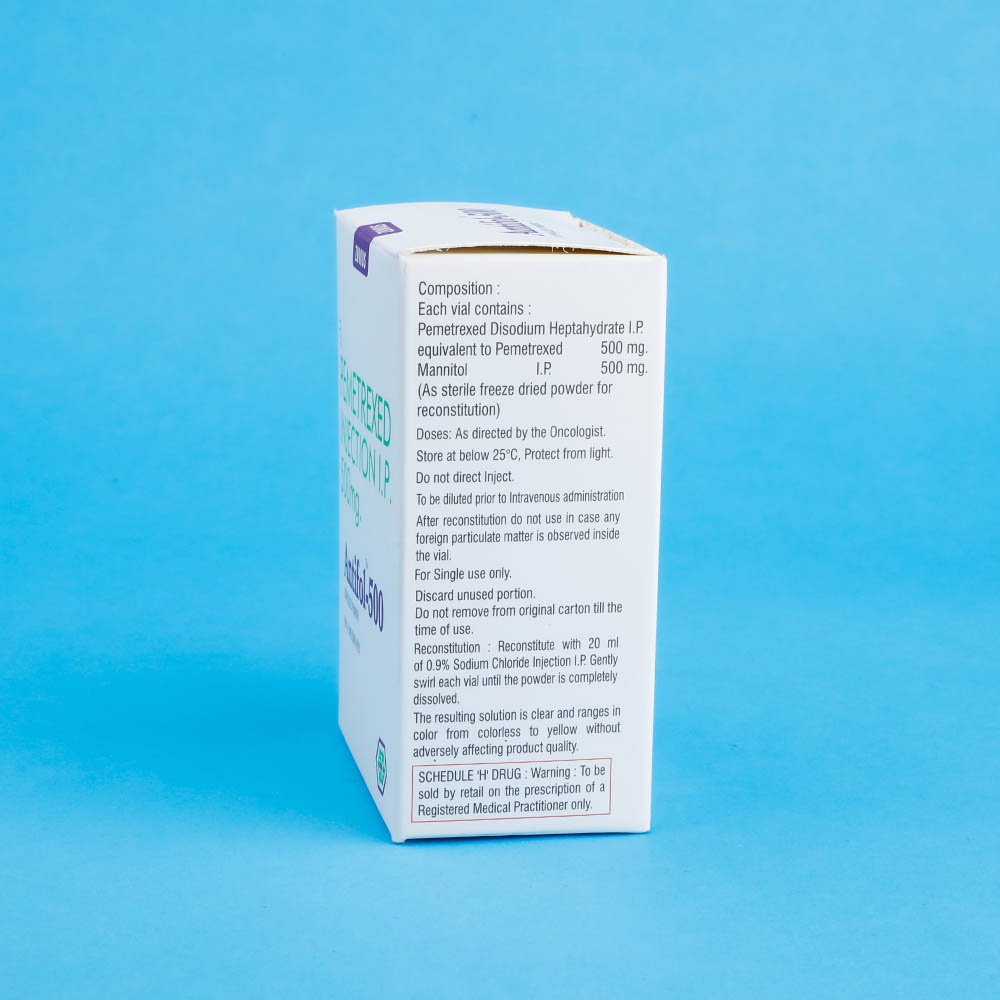
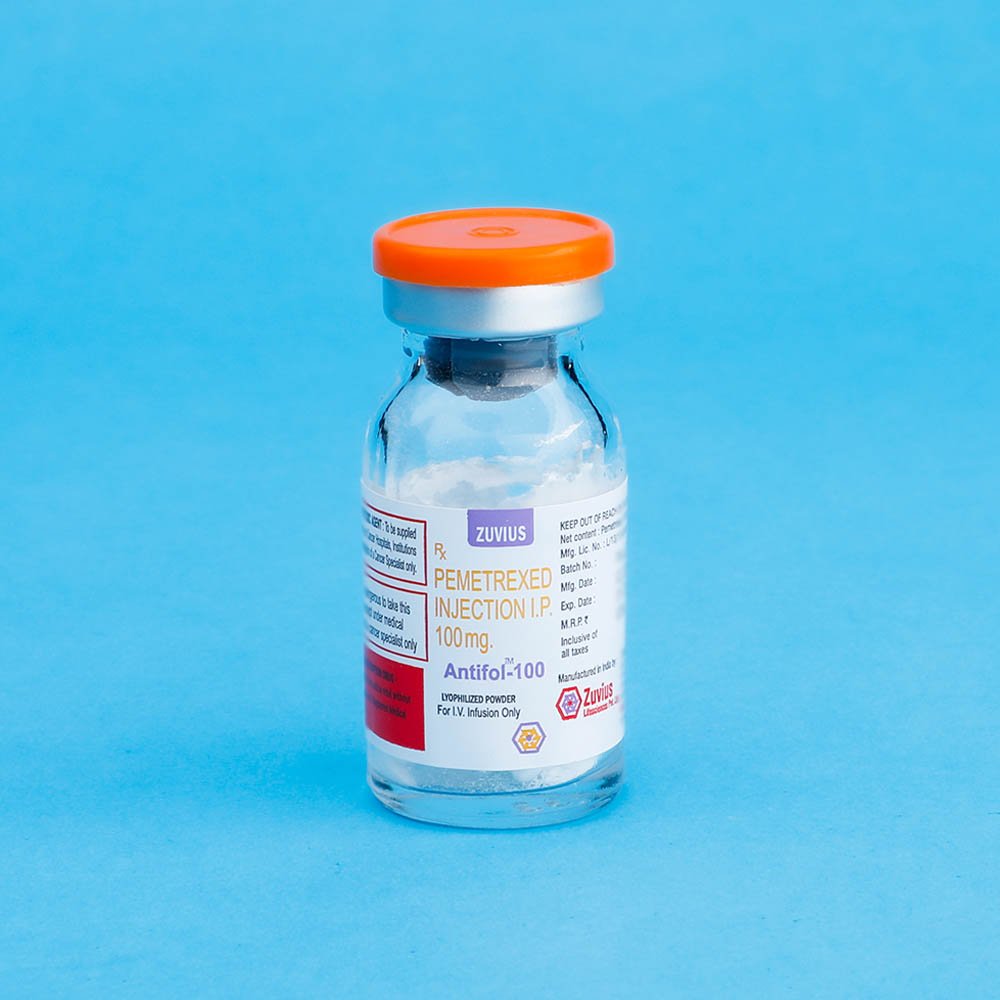
Antifol- Pemetrexed Inj 500 mg
Pemetrexed
Strength: 100mg / 500mg
Pack Size: 1 vial
Drug Class: Folic acid analogues
Dosage and Administration:
- Combination Use with Cisplatin for Nonsqamous Non-Small Cell Lung Cancer or Malignant pleural mesothelioma
The recommended dose of Pemetrexed is 500 mg/m2infused over 2 hours beginning approximately 30 minutes after the end of the cycle. The recommended dose of cisplatin is 75 mg/m2infused over 2 hours beginning approximately 30 minutes after the end of Pemetrexed administration.
Single-Agent Use as Maintenance Following First-Line Therapy, or as a Second-Line Therapy The recommended dose of Pemetrexed is 500 mg/m2 administered as an intravenous infusion over 10minutes on Day 1 of each 21-day cycle.
- Premedication Regimen and Concurrent Medications
- Vitamin Supplementation
Folic acid 400 mcg to 1000 mcg orally to be initiated once daily, beginning 7 days before the first
The dose of Pemetrexed. Continue folic acid during the full course of therapy and for 21 days the last dose of Pemetrexed.
Vitamin B12 1mg intramuscularly 1 week prior to the first dose of Pemetrexed and every 3 cycles thereafter to be administered. Subsequent vitamin B12 injections may be given the same day as treatment with Pemetrexed.
- Corticosteroids
Administer dexamethasone 4mg by mouth twice daily the day before, the day of, and the day after Pemetrexed administration.
- Laboratory monitoring and dose reduction/discontinuation recommendations
- Monitoring: Complete blood cell counts, including platelet counts, should be performed on all patients receiving Pemetrexed. Patients should be monitored for nadir and recovery, which were tested in the clinical study before each dose and on days 8 and 15 of each cycle. Patients should not begin a new cycle of treatment unless the ANC is = 1500 cells/mm3, the platelet count is = 100,000 cells/mm3 and creatinine clearance is = 45mL/min. Periodic chemistry tests should be performed to evaluate renal and hepatic function.
- Dose Reduction Recommendations: Dose adjustments at the start of a subsequent cycle should be based on nadir hematologic counts or maximum nonhematologic toxicity from the preceding cycle of therapy. Treatment may be delayed to allow sufficient time for recovery. Upon recovery, patients should be retreated using the guidelines in Tables 1-3, which are suitable for using Pemetrexed as a single-agent or in combination with cisplatin.
Table 1: Dose Reduction for Pemetrexed (single-agent or in combination) and Cisplatin- Hematologic Toxicities
| Nadir ANC < 500/mm3 and nadir platelets 50,000/ mm3 | 75% of previous dose (Pemetrexed and cisplatin). |
| Nadir platelets < 50,000/ mm3without bleeding regardless of nadir ANC. | 75% of previous dose (Pemetrexed and cisplatin). |
| Nadir platelets < 50,000/ mm3without bleeding regardless of nadir ANC. | 50% of previous dose (Pemetrexed and cisplatin). |
| *These criteria meet the CTC version 2.0 (NCI 1998) definition of = CTC Grade 2 bleeding. | |
If patients develop nonhematologic toxicities (excluding neurotoxicity)=Grade 3, treatment should be withheld until resolution to less than or equal to the patient’s pre-therapy value. Treatment should be resumed according to guidelines in Table 2.
Table 2: Dose Reduction for Pemetrexed (single-agent or in combination) and Cisplatin-Nonhematologic Toxicities
| DOSE OF Pemetrexed (MG/M2) | DOSE OF CISPLATIN (MG/M2) | |
| Any Grade 3 or 4 toxicities except mucositis | 75% of previous dose | 75% of previous dose |
| Any diarrhea requiring hospitalization (irrespective of Grade) or Grade 3 or 4 diarrhea | 75% of previous dose | 75% of previous dose |
| Grade 3 or 4 mucositis | 50% of previous dose | 100% of previous dose |
| a NCI Common Toxicity Criteria (CTC) | ||
| b Excluding neurotoxicity (see Table 3). |
in the event of neurotoxicity, the recommended dose adjustments for Pemetrexed and cisplatin are described in Table 3. Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is experienced.
Table 3: Dose Reduction for Pemetrexed (single-agent or in combination) and Cisplatin – Neurotoxicity
| CTCGRADE | DOSE OF Pemetrexed (MG/M2) | DOSE OF CISPLATIN (MG/M2) |
| 0-1 | 100% of previous dose | 100% of previous dose |
| 2 | 100% of previous dose | 50% of previous dose |
Discontinuation Recommendation:
Pemetrexed therapy should be discontinued if a patient experiences any hematologic or nonhematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if grade 3 or 4 neurotoxicity is observed.
Renal Impaired Patients:
In clinical studies, patients with creatinine clearance = 45 ml/min required no dose adjustments other than those recommended for all patients. Insufficient numbers of patients with creatinine clearance below 45 ml/min have been treated to make dosage recommendations for this group of patients. Therefore, Pemetrexed should not be administered to patients whose creatinine clearance is < 45ml/min using the standard Cockcroft and Gault formula (below) or GFR measured by Tc99m-DTPA serum clearance method:
| (weight in kg) x (140-age) | |
| Males : | (72) x serum creatinine (mg/100ml.) |
| Females : | estimated creatinine clearance for males x 0.85 |
Caution should be exercised when administering Pemetrexed concurrently with NSAIDs to patients whose creatinine clearance is <80 ml/min
USE IN SPECIFIC POPULATIONS
i. Pregnancy-Teratogenic Effects-Pregnancy Category D
Embryotoxicity was characterized by increased embryo-fetal deaths and reduced litter size. If Pemetrexed is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
ii. Nursing Mothers
it is not known whether Pemetrexed or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from Pemetrexed, a decision should be made to discontinue nursing or discontinue the drug, taking into account the importance of the drug for the mother.
iii. Pediatric Use
The efficacy of Pemetrexed in pediatric patients has not been demonstrated. The most common toxicities reported were hematological (Leukopenia, Neutropenia / Granulocytopenia, Anemia, thrombocytopenia, and Lymphopenia), liver function abnormalities (increased ALT/AST), fatigue, and nausea.
iv. Geriatric Use
Pemetrexed is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Renal fusion monitoring is recommended with the administration of Pemetrexed. No dose reductions other than those recommended for all patients are necessary for patients 65 years of age or older.
v. Patients with Hepatic Impairment
There was no effect of elevated AST, ALT, or total bilirubin on the pharmacokinetics of Pemetrexed.
Cold Storage: no
Pemetrexed disodium heptahydrate has the chemical name L-Glutamine acid, N-[4-[2-(2-amino-4,7-dihydro-4-oxo1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-,disodium salt, heptahydrate. It is a white to almost-white solid with a molecular formula of C20H19N5Na2O67H2O and a molecular weight of 597.49. The Structural formula is as follows:
A. Nonsquamous Non-Small Cell Lung Cancer-Combination with Cisplatin
Pemetrexed is indicated in combination with cisplatin therapy for the initial treatment of patients with locally advanced or metastatic nonsquamous non-small cell lung cancer.
B. Nonsquamous Non-Small Cell Lung Cancer-Maintenance
Pemetrexed is indicated for the maintenance treatment of patients with locally advanced or metastatic nonsquamous non-small cell lung cancer whose disease has not progressed after four cycles of platinum-based first-line chemotherapy.
C. Nonsquamous Non-Small Cell Lung Cancer – After Prior Chemotherapy
Pemetrexed is indicated as a single-agent for the treatment of patients with locally advanced or metastatic nonsquamous non-small cell lung cancer after prior chemotherapy.
D. Mesothelioma
Pemetrexed in combination with cisplatin is indicated for the treatment of patients with malignant pleural mesothelioma whose disease is unresectable or who are otherwise not candidates for curative surgery.
Limitations of Use Pemetrexate is not indicated for the treatment of patients with squamous cell non-small cell lung cancer.
Pemetrexed is a folate analog metabolic inhibitor that disrupts folate-dependent metabolic processes essential for cell replication. Pemetrexed is taken into cells by membrane carriers, such as the reduced folate carrier and membrane folate binding protein transport systems. Pemetrexed continuation maintenance chemotherapy is active and well tolerated. Pemetrexed maintenance should be considered in patients with advanced adenocarcinoma lung patients who have not progressed on completion of induction chemotherapy.
- Read the label carefully before use
- Store below 25°C. Protect from light. Do not direct inject.
- Keep out of the reach of children
Each 100mg vial to be reconstituted with 4.22 ml of 0.9% (w/v) Sodium Chloride Injection IP and gently mixed so that the powder is completely dissolved.
Each 500mg vial to be reconstituted with 20ml of 0.9% (w/v) Sodium Chloride IP and gently mixed so that the powder is complety dissolved. The resulting solution is clear and ranges in color from colorless to yellow or green-yellow chemical and physical stability of reconstituted and infusion solutions of Pemetrexed were demonstrated for up to 24 hours following initial reconstitution, when stored refrigerated, 2-8°C (36-46°F). When prepared as directed, reconstituted and infusion solutions of Pemetrexed contain no antimicrobial preservatives. Unused portion is discarded. Pemetrexed is not light sensitive.
Each 100mg vial to be reconstituted with 4.22 ml of 0.9% (w/v) Sodium Chloride Injection IP and gently mixed so that the powder is completely dissolved.
Each 500mg vial to be reconstituted with 20ml of 0.9% (w/v) Sodium Chloride IP and gently mixed so that the powder is complety dissolved. The resulting solution is clear and ranges in color from colorless to yellow or green-yellow chemical and physical stability of reconstituted and infusion solutions of Pemetrexed were demonstrated for up to 24 hours following initial reconstitution, when stored refrigerated, 2-8°C (36-46°F). When prepared as directed, reconstituted and infusion solutions of Pemetrexed contain no antimicrobial preservatives. Unused portion is discarded. Pemetrexed is not light sensitive.
Each 100mg vial to be reconstituted with 4.22 ml of 0.9% (w/v) Sodium Chloride Injection IP and gently mixed so that the powder is completely dissolved.
Each 500mg vial to be reconstituted with 20ml of 0.9% (w/v) Sodium Chloride IP and gently mixed so that the powder is completely dissolved. The resulting solution is clear and ranges in color from colorless to yellow or green-yellow chemical and physical stability of reconstituted and infusion solutions of Pemetrexed were demonstrated for up to 24 hours following initial reconstitution, when stored refrigerated, 2-8°C (36-46°F). When prepared as directed, reconstituted and infusion solutions of Pemetrexed contain no antimicrobial preservatives. The unused portion is discarded. Pemetrexed is not light sensitive.