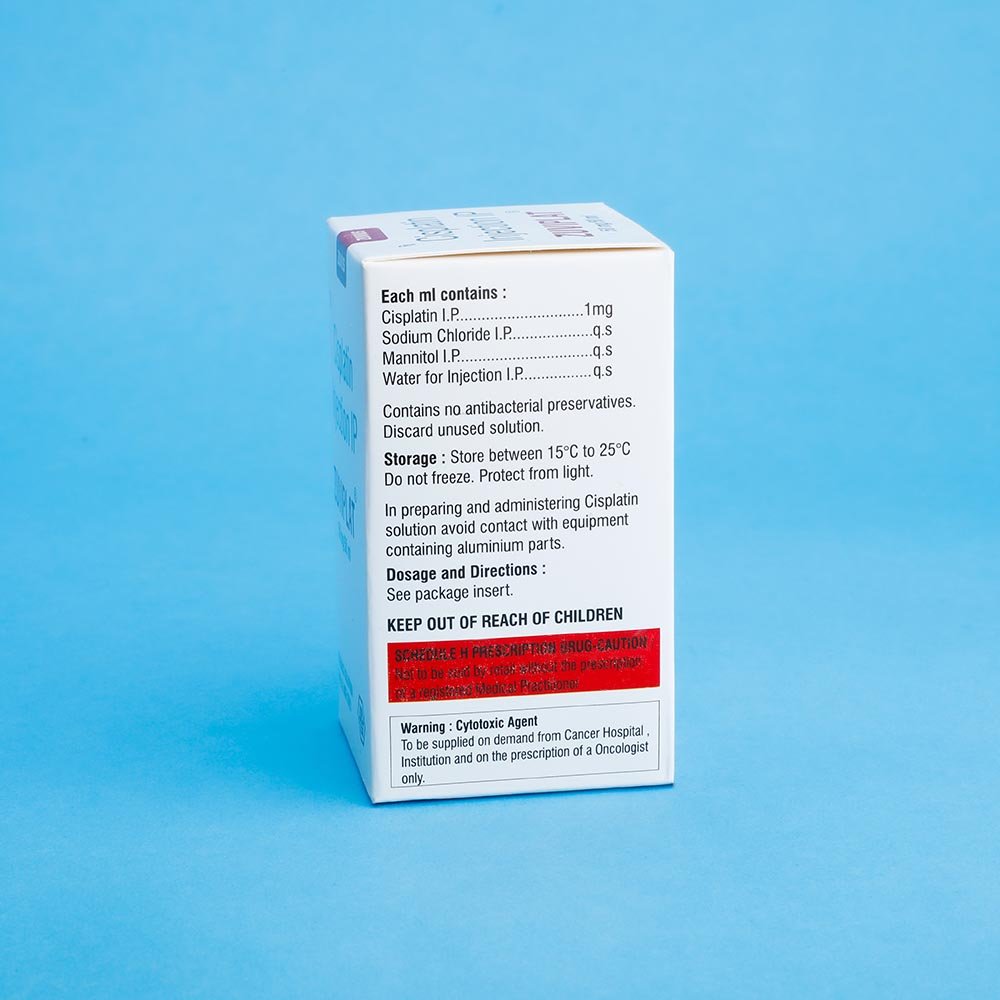
Zuviplat- Cisplatin Inj
Cisplatin
Strength: 10 mg / 50mg
Pack Size: 1 vial
Drug Class: Antineoplastic agents / Platinum compounds
Dosage and Administration:
ZUVIPLAT solution should be used intravenously only and should be administered by i.v dose every 3-4 week. An alternative regimen is 15-20 mg per sq. i.v daily for 5 days every 3-4 week In clinical use of ZUVIPLAT injection, take the following measure for reducing the nephrotoxicity of the drug: Administer 1000-2000 ml. of an adequate fluid deficit replacement over 4 hours or more prior to the administration of ZUVIPLAT injection. Administer ZUVIPLAT injection as a mixture with 500-1000 ml of physiological saline or a dextrose –saline solution by intravenous drip over 2 hours or more. When it take the intravenous drip over 2 hours or more. When it take the intravenous drip of ZUVIPLAT injection a long time, keep the dripping bottle away from light. After the administration of ZUVIPLAT injection, administer 1000-2000 ml of an adequate fluid deficit replacement over 4 hours or more. During the administration of ZUVIPLAT injection, exercise care so as to maintain the urinary output and administer diuretic such as mannitol and furosemide.
Cold Storage: no
ZUVIPLAT is an anticancer drug of an entirely new category for antitumor activity and low toxicity from among a variety platinum compound developed according to the discovery by Barnett Rosenberg in the U.S.A that platinum compound inhibit the mitosis of Escherichia coli.
ZUVIPLAT can be used itself or in addition to other modalities or preferable in established combination therapy with other approved chemotherapeutic agent. It is indicated in metastic ovarian turnovers, squamous cell carcinoma of the head and neck and advance bladder carcinoma.
Cisplatin is considered as one of the most effective anticancer drugs used widely for the treatment of solid tumors. One of the most prominent and effective clinical applications of cisplatin is on the treatment of lung cancer.
PRECAUTION SPECIAL:
Patients with renal disorder
Patients with hepatic disorder
Patients with myelosuppression
Patients with auditory disorder.
ZUVIPLAT solution should be drawn up out of the vial using a syringe and sterile technique and added to 2 liters of either a 5% dextrose in1/3 normal saline solution, or 5% dextrose in a normal saline solution. 37.5 gm of mannitol should also be added in the infusion solution. As with other potentially toxic compounds. Caution in handling the solution of ZUVIPLAT should be exercised. Skin reactor associated with accidental exposure to ZUVIPLAT may occur. The use of the gloves is recommended. If ZUVIPLAT solution comes in the contract with skin or mucosa, immediately wash thoroughly with soap and water. Do not mix ZUVIPLAT injection with 5% dextrose, amino acid solution or any solution containing sodium locate for administration for intravenous drip, because ZUVIPLAT may be degraded in such a mixture. Because ZUVIPLAT react with aluminumto form precipitate, resulting in deteriorated activity Because ZUVIPLAT is a chelate compound, ZUVIPLAT injection should not be mixed with any other anticancer drugs ZUVIPLAT injection when mixed with physiological saline or a dextrose- saline solution, should be administered as early as possible Because ZUVIPLAT is degraded by light keep ZUVIPLAT injection away from direct sunlight When it take the intravenous drip of PLATINEX injection a long time keep the dripping bottle away from light
OTHER PRECAUTION:
It has been report that the administration of ZUVIPLAT injection may in some cases be associated with congestive heart failures and abnormal ECG, and also with hypomagnesaemia and hypocalcemia 3
ZUVIPLAT has proved mutagenic in bacteria
Its has been reported that the intra peritoneal administration of ZUVIPLAT to mice caused pulmonary adenoma and skin turnover
USE IN PREGNANCY:
ZUVIPLAT is carcinogenic ans should be considered teratogenic. Termination of pregnancy (especially first trimester) should be considered in patients given ZUVIPLAT
Cisplatin may cause serious kidney problems. Tell you doctor right away if you have blood in urine, change in frequency of urination or amount of urine, difficulty in breathing, drowsiness, increased thirst, loss of appetite, nausea or vomiting, swelling of the feet or lower legs, or weakness.