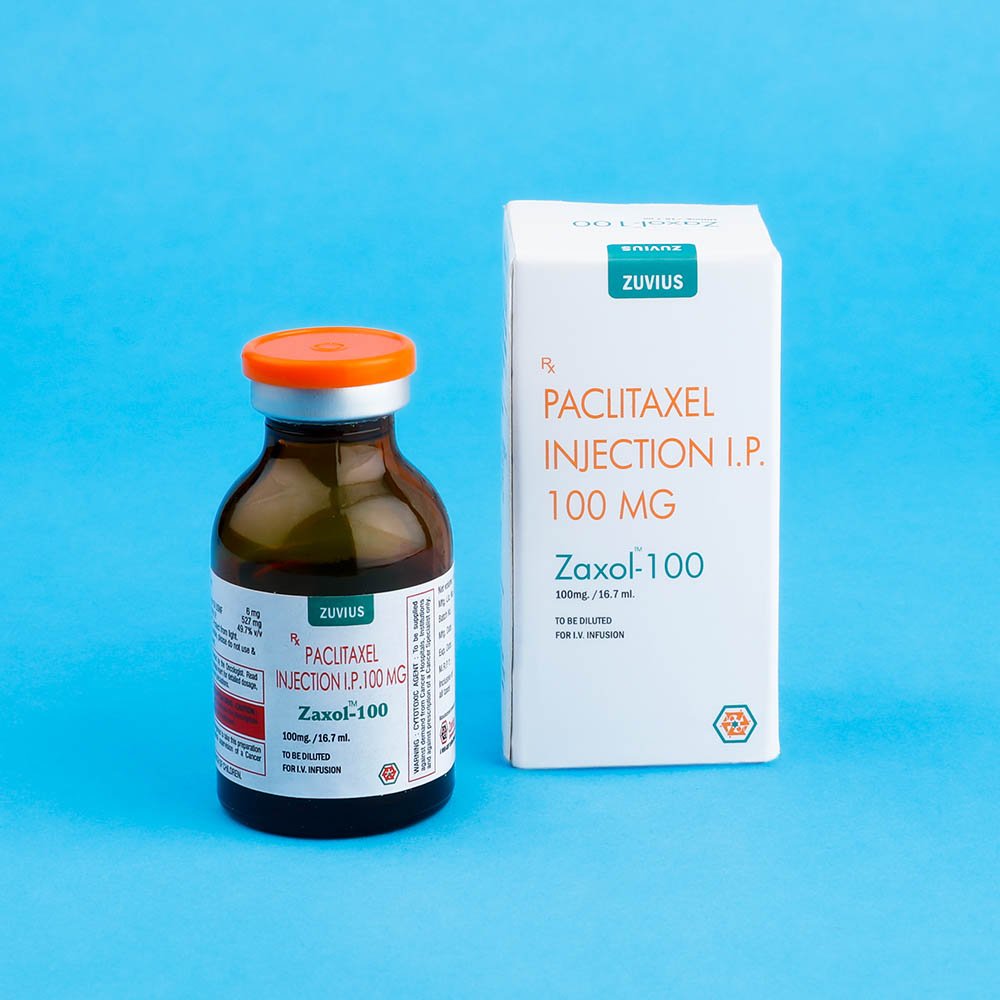
Zaxol- Paclitaxel Inj
Paclitaxel
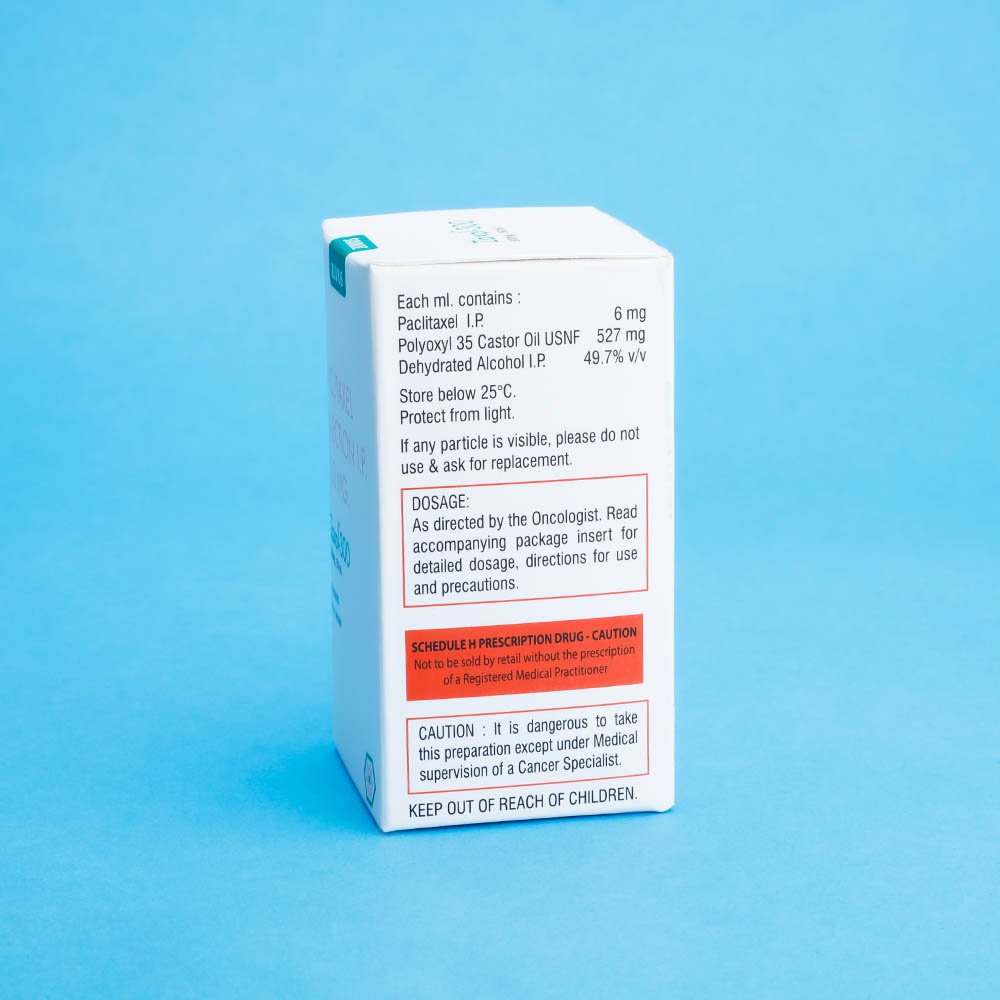
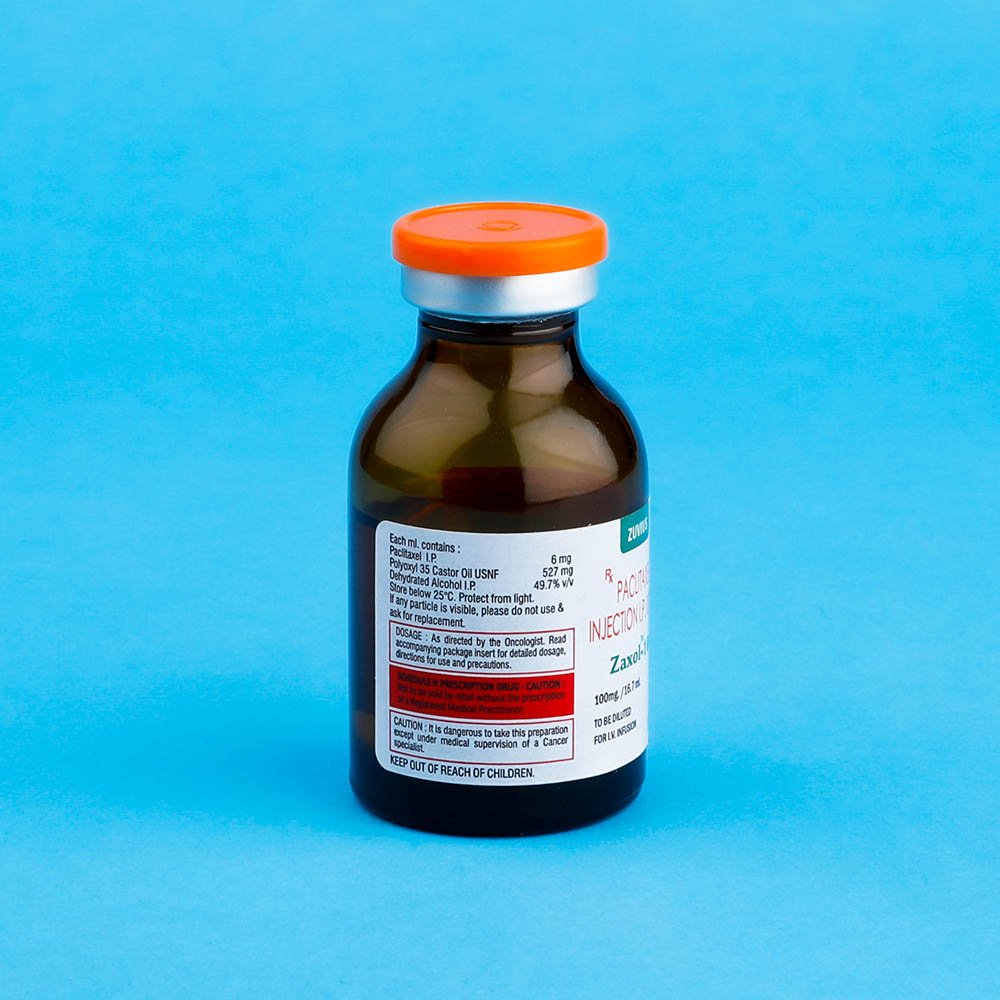
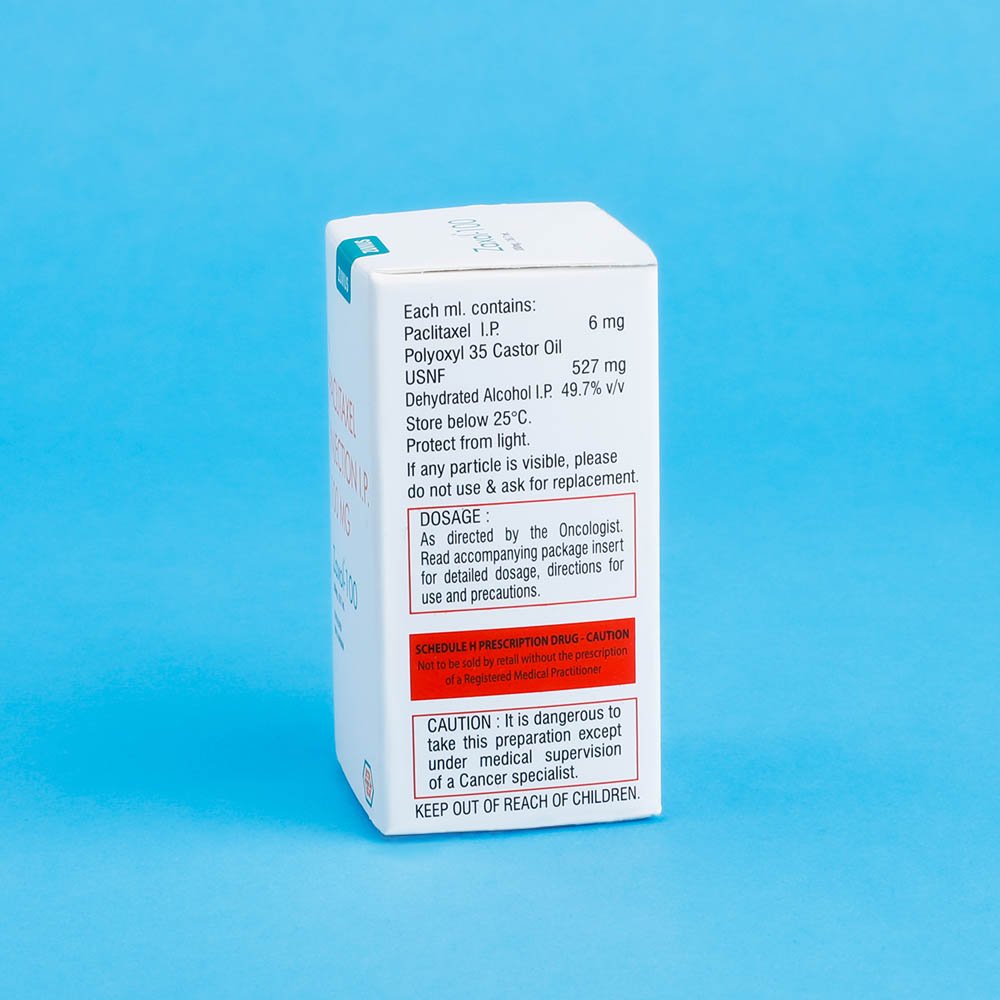
Strength: 30mg / 100mg / 260mg / 300mg
Pack Size: 1 vial
Drug Class: Antineoplastic agent/taxanes
Dosage and Administration:
All patients should be per medicated prior to ZAXOL administration in order to minimize severe hypersensitivity reaction Such pre medication may consist of dexamethasone 20mg orally approximately 12 and 6 hours before ZAXOL, diphenhydramine (or its equivalent) 50mg IV 30 to 60 minutes prior to ZAXOL and cimetidine (300mg) or rantidine (50mg) IV 30 to 60 minutes before ZAXOL
ZAXOL at a dose of 175mg/m2 administration intravenously. Over 3 hours every three week has been to the effective in patientswith metastatic carcinoma of the ovary or breast who have failed standard therapy. Single course of ZAXOL should not be repeated until the neutrophil count is at least 1500 cells/mm2 and platelet count is at least 100,000 cells/mm2 patient who experience who experience severe neutropenia (neutrophil <500 cells/mm2) or severe peripheral neutropenia during ZAXOL therapy should have the dosage reduced by 20% for subsequent course of ZAXOL
Preparation for Intravenous Administration
ZAXOL for injection must be diluted prior to infusion. ZAXOL should be diluted in 0.9% sodium chloride injection, 5% dextrose injection, 5% dextrose and 0.9% sodium chloride injection, or 5 % dextrose in Ringer’s injection to a final concentration of 0.3 to 1.2mg/ml. The solution are physically and chemically stable for up to 27 hours at ambient temperature (15 – 30ᴼ). Upon preparation solution may show haziness, which is attributed to the formulation vehicle.
No significant loss in potency has been noted following simulated delivery of the solution through I.V. tubing containing an in – line (0.22 micron) filter
Data collected for the presence of the extractable plasticizer DEPH (di(2-exthylexly phthalate) show that level increase with time and concentration when dilutions are prepared in pvccontainers. Consequently the use of plasticized pvc containers and administration sets are not recommended. ZAXOL solution should be prepared and stored in glass, polypropylene, or polyolefin containers Non-PVC containing administration sets, such as those which are polyethylene- lined, should be used.
Cold Storage: no
Paclitaxel is a natural product with antitumor activity. Paclitaxel is obtained via a semisynthetic process from Taxusbaccata. The chemical name for paclitaxel is 5B, 20-Epoxy 1,2 a, 4,7B, 10 B, 13 a-hexahydroxytax-11-en-9-one 4,10-diacetate 2-benoate 13-ester with (2R,. 3S)-N-benzoyl-3-phenylisoserine.
Paclitaxel has the following structural formula:
Paclitaxel is a white to off-white crystalline powder with the empirical formula C47H51 NO14 and a molecular weight of 853.9. It is highly lipophilic, insoluble in water, and melts at around 216-217°C.
ZAXOL (Paclitaxel) is indicated for the treatment of metastatic carcinoma of the ovary or breast after failure of standard therapy. The standard therapy means anthracycline containing regimen for ovarian cancer unless clinically contraindicated.
Usage:
As directed by the Oncologist.
Paclitaxel is an antimicrotubule agent that promotes the assembly of microtubules from tubulin dimers and stabilises microtubules by preventing depolymerisation. This stability inhibits the normal dynamic reorganisation of the microtubule network, which is essential for vital interphase and mitotic cellular functions. In addition, paclitaxel induces abnormal arrays or bundles of microtubules throughout the cell cycle and multiple asters of microtubules during mitosis. Paclitaxel helps in improving bone metastasis of lung cancer and has an important significance in clinical treatment of bone metastasis of lung cancer.
- Read the label carefully before use
- Store in a cool and dry place, away from sunlight
- Keep out of the reach of children
During Paclitaxel therapy, continuous cardiac monitoring should be performed in patients with a previous history of conduction abnormalities since cardiac adverse effects (i.e. Myocardial infarction symptoms of congestive heart failure) have been reported in patients receiving Paclitaxel.
Paclitaxel produces severe dose-limiting bone marrow depression. Therefore regular blood counts should be performed on patients receiving Paclitaxel, and dosage should not be repeated until the neutrophils count is greater than 1,500 cell/mm2 and the platelet count is at least /greater the 100,000cell/mm2
Interactions With other Drugs
Concurrent use of radiation therapy with Paclitaxel should be avoided because of additive bone marrow depression. Pretreatment with Cisplatin may reduce the clearance of Paclitaxel resulting in increased toxicity specially increased myelosuppression. Hence, when the two drugs are given in combination Paclitaxel should be given first.
The metabolism of Paclitaxel is catalyzed by CYP2C8 and CYP3A4 in the absence of drug interaction data caution should be exercised when administering Paclitaxel concomitantly with known substrates or inhibitors of Cytochrome P450 isoenzymes CYP2CB and CYP3A4.
Paclitaxel should be given with caution to those patients who are on Ketoconazole therapy due to the inhibition of Paclitaxel metabolism by Ketoconazole leading to drug accumulation.