Capetaz Tab
Capecetabine Tab
Strength: 500mg
Pack Size: 1 x 10
Drug Class: Cytostatic (antimetabolite)
Dosage and Administration:
The recommended dose of Capecitabine is 2500 mg /m2 administered orally daily with food for 2weeks followed by a 1 week rest period given as 3 week cycles. The Capecitabine daily dose should be given orally in two divided doses (approximately 12 hours apart) at the end of a meal. Capecitabine tablets should be swallowed with water. The following table displays the total daily dose by body surface area and the number of tablets to be taken at each dose.
| Capecitabine Dose Calculation According to Body Surface Area | |||
| Dose level 225 mg/m2/day | Number of tablets to be taken at each dose (morning and evening) | ||
| Surface Area (m2) | Total Daily* Dose (mg) | 150 mg | 500mg |
| </= 1.24 | 3000 | 0 | 3 |
| 1.25-1.36 | 3300 | 1 | 3 |
| 1.37-1.51 | 3600 | 2 | 3 |
| 1.52-1.64 | 4000 | 0 | 4 |
| 1.65-1.76 | 4300 | 1 | 4 |
| 1.65-1.76 | 4600 | 2 | 4 |
| 1.92-2.04 | 5000 | 0 | 5 |
| 2.05-2.17 | 5300 | 1 | 5 |
| >/=2.18 | 5600 | 2 | 5 |
*Total Daily dose divided by 2 to allow equal morning and evening doses.
Dose Modification guidelines: Patients should be carefully monitored for toxicity. Toxicity due to Capecitabine administration may be managed by symptomatic treatment, dose interruptions and adjustment of Capecitabine dose. Once dose has been reduced it should not be increased at a later time.
| Recommended Dose Modifications | ||
| Toxicity NCCIC Grades* | During a Course of Therapy | Dose Adjustment for Next Cycle (% of starting dose) |
| Grade 1 | Maintain dose level | Maintain dose level |
| Grade 2 | ||
| 1st appearance | Interrupt until resolved to grade 0-1 |
100% |
| 2nd appearance | Interrupt until resolved to grade 0-1 |
75% |
| 3rd appearance | Interrupt until resolved to grade 0-1 |
50% |
| 4th appearance | Discontinue treatment permanently |
|
| Grade 3 | ||
| 1st appearance | Interrupt until resolved to grade 0-1 |
75% |
| 2nd appearance | Interrupt until resolved to grade 0-1 |
50% |
| 3rd appearance | Discontinue treatment permanently |
|
| Grade 4 | ||
| 1st appearance | Discontinue permanently or if physician deems it to be in the patient’s best interest to continue, interrupt until resolved to grade 0-1 | 50% |
*National Cancer Institute of Canada Common Toxicity Criteria were used except for the Hand-and-Doot syndrome.
Adjustment of Starting Dose In Special Populations: Hepatic Impairment: In patient with mild to moderate hepatic dysfunction due to liver metastases, no starting dose adjustment tis necessary however, patients should be carefully monitored. Patients with severe hepatic dysfunction have not been studied.
Renal Important: Insufficient data are available in patients with renal impairment to provide a dosage recommendation.
Geriatric population: The elderly may be pharmcodynamically more sensitive to the toxic effects of 5-Fu and therefore, physician should exercise caution in monitoring the effects of Capecitabine in the elderly. Insufficient data are available to provide a dosage recommendation.
Cold Storage: no
CAPETAZ 500 tablets containing Capecitabine 500mg are Peach colored capsule shaped film coaed tablets. Capecitabine is a fluoropyrimidinecarbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5’-deoxy-5-fluorouridine (5’DFUR) which is converted to 5-fluorouracil.
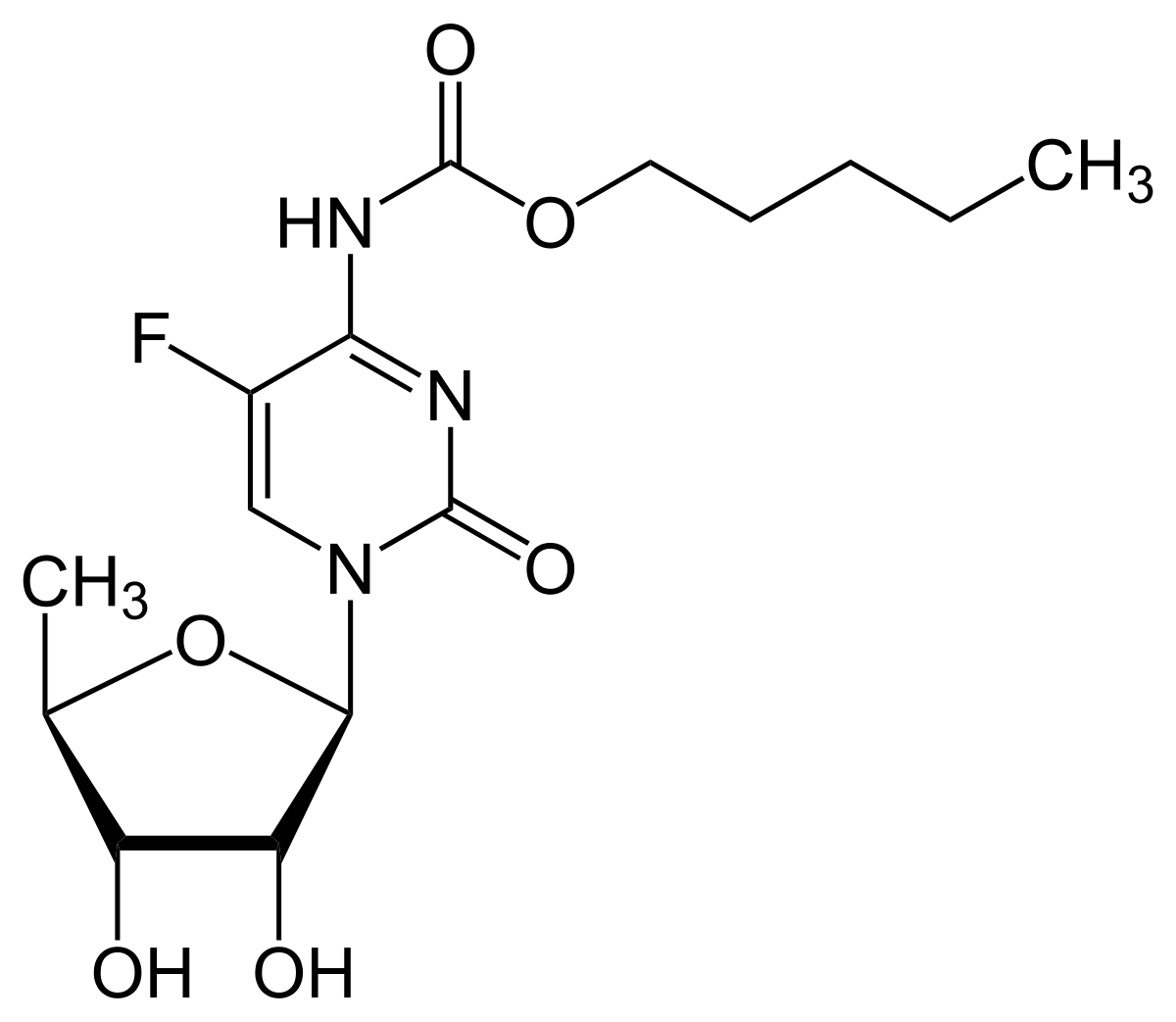
The chemical name for capecitabine is 5’-deoxy-5-fluoro-N-[-(pentyloxy) carbonyl]-cytidine and has a molecular weight of 359.35. capecitabine has the following structural formula:
Capecitabine is indicated for the treatment of patients with metastatic breast cancer resistant to both paclitaxel and an anthracycline-containing chemotherapy regimen or resistant to paclitaxel and for whom further anthracycline therapy is not indicated.
for the adjuvant treatment of patients following surgery of stage III (Dukes’ stage C) colon cancer.
– for the treatment of metastatic colorectal cancer.
– for first-line treatment of advanced gastric cancer in combination with a platinum based regimen.
– in combination with docetaxel for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline.
Enzymes convert capecitabine to 5-fluorouracil (5-FU) in vivo. Both normal and tumor cells metabolize 5-FU to 5-fluoro-2′-deoxyuridine monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). These metabolites cause cell injury by two different mechanisms. First, FdUMP and the folate cofactor,
N5-10 -methylenetetrahydrofolate, bind to thymidylate synthase (TS) to form a covalently bound ternary complex. This binding inhibits the formation of thymidylate from 2′-deoxyuridylate. Thymidylate is the necessary precusor of thymidine triphosphate, which is essential for the synthesis of DNA, so that a deficiency of this compound can inhibit cell division. Second, nuclear transcriptional enzymes can mistakenly incorporate FUTP in place of uridine triphosphate (UTP) during the synthesis of RNA. This metabolic error can interfere with RNA processing and protein synthesis.
In repeat-dose toxicity studies, daily oral administration of capecitabine to cynomolgus monkeys and mice produced toxic effects on the gastrointestinal, lymphoid and haemopoietic systems, typical for fluoropyrimidines. These toxicities were reversible. Skin toxicity, characterised by degenerative/regressive changes, was observed with capecitabine. Capecitabine was devoid of hepatic and CNS toxicities. Cardiovascular toxicity (e.g. PR- and QT-interval prolongation) was detectable in cynomolgus monkeys after intravenous administration (100 mg/kg) but not after repeated oral dosing (1379 mg/m2/day).
A two-year mouse carcinogenicity study produced no evidence of carcinogenicity by capecitabine.
During standard fertility studies, impairment of fertility was observed in female mice receiving capecitabine; however, this effect was reversible after a drug-free period. In addition, during a 13-week study, atrophic and degenerative changes occurred in reproductive organs of male mice; however these effects were reversible after a drug-free period.
In embryotoxicity and teratogenicity studies in mice, dose-related increases in foetal resorption and teratogenicity were observed. In monkeys, abortion and embryolethality were observed at high doses, but there was no evidence of teratogenicity.
Capecitabine was not mutagenic in vitro to bacteria (Ames test) or mammalian cells (Chinese hamster V79/HPRT gene mutation assay). However, similar to other nucleoside analogues (ie, 5-FU), capecitabine was clastogenic in human lymphocytes (in vitro) and a positive trend occurred in mouse bone marrow micronucleus tests (in vivo).
A physician experienced in the use of cancer chemotherapeutic agents should monitor patients reciving therapy with Capecitabine. Most adverse events are reversible and do not need to result in discontinuation, although doses may need to be with held or reduced.
Hand-and-Foot Syndrome: Hand-and-foot syndrome (palmar-plantar erythrodysesthesia or chemotherapy induced acral erythema) may occurs, administration of Capecitabine should be interrupted until the event resolve or decreases in intensity to grade 1. Following grade 3 hand-and-foot syndrome, subsequent doses of Capecitabine should be decreased.
Cardiac: There has been cardiotoxicity associated with fluorinatedpyrimidine therapy, including myocardial infarction, angina, dysrhythmias, cardiogenic shock, sudden death and electrocardiograph changes. These adverse events may be more common in patients with a prior history of coronary artery disease.
Hepatic Insufficiency: patients with mild to moderate hepatic dysfunction due to liver metastases should be carefully monitored when Capecitabine is administered. The effect of severe hepatic dysfunction on the disposition of Capecitabine is not known.
Hyperbilirubinemia: If drug related grade 2-4 elevations in bilirubin occur, administration of Capecitabine should be immediately interrupted until the Hyperbilirubinemia resolves or decreases in intensity to grade 1.
Renal Insufficiency: There is little experience in patients with renal impairment. Physicians should exercise caution when Capecitabine is administered.
Hematologic: Capecitabine can lead neutropenia, thrombocytopenia and decreases in hemoglobin.
Carcinogenesis, Mutagenesis & Impairment of Fertility
Carcinogenesis and Mutagenesis: Long – term studies in animals to evaluate the carcinogenic potential of Capecitabine have not been conducted. Capecitabine has not been shown to be mutagenic in vitro or in vivo.
Impairment of Fertility: Capecitabine causes a decrease in fertility by disturbing the estrus. In male mice, Capecitabine causes degenerative changes in the testes, including decreases in the number of spermatocytes and spermatids.
Nursing Women: It is not known whether the drug is excreted in human milk. Because many durgs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants. It is recommended that nursing be discontinued when receiving Capecitabine therapy.
Pediatric Use: The safety and effectiveness of Capecitabine in persons <18 years of age have not been established.
Geriatric Use: Patients >/=80 years old may experience a greater incidence of gastrointestinal grade 4 or 4 adverse events. Physicians should pay particular attention to monitoring the adverse effects of Capecitabine in the elderly.
Drug-Food Interaction: Since current safety and efficacy data are based upon administration of Capecitabine with food, it is recommended that Capecitabine be administered with food.