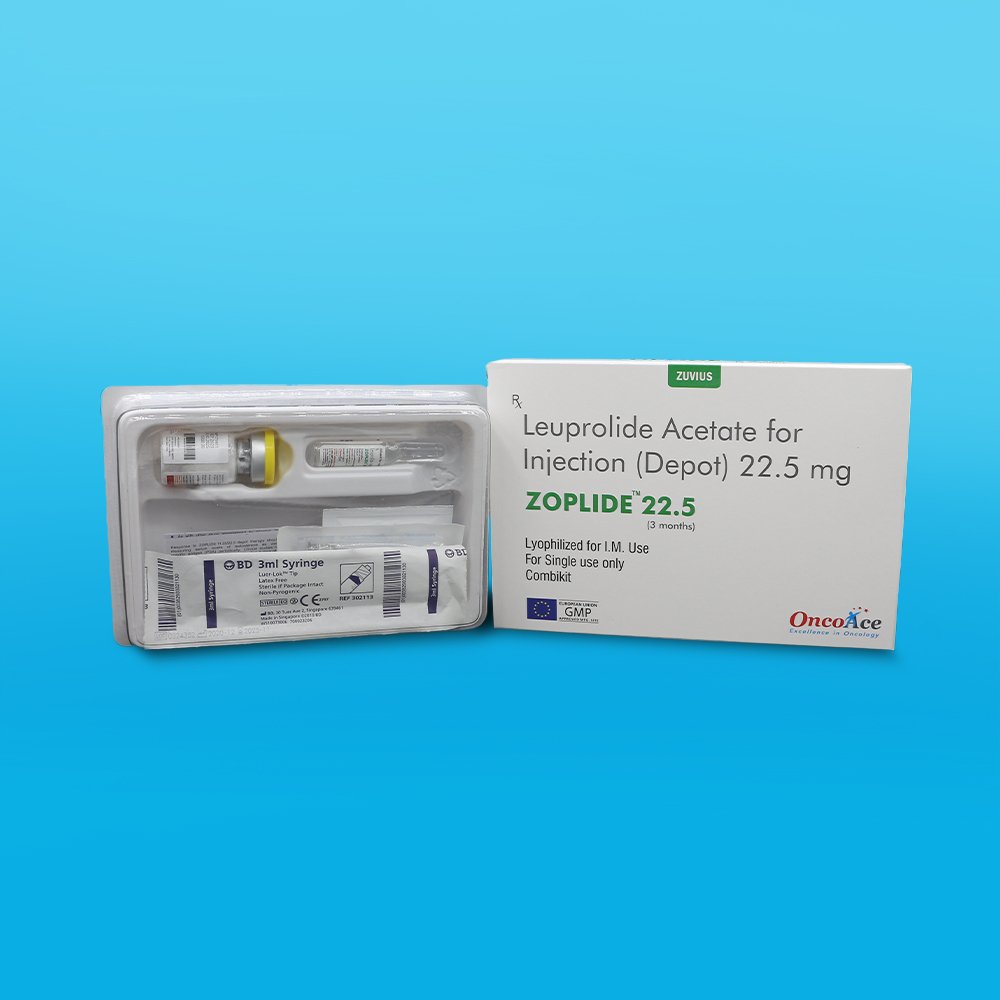
Zoplide- Leuprolide Acetate Inj
Leuprolide Acetate
Strength: 3.75, 11.25, 22.50 mg
Pack Size: 1 Vial
Drug Class: Gonadotropin-releasing hormone (GnRH) agonists
Dosage and Administration:
The recommended dose is 1 mg (0.2 mL or 20 unit mark) administered as a single daily
subcutaneous injection. As with other drugs administered chronically by subcutaneous
injection, the injection site should be varied periodically. Each 0.2 mL contains 1 mg of
leuprolide acetate, sodium chloride for tonicity adjustment, 1.8 mg of benzyl alcohol as
preservative and water for injection. The pH may have been adjusted with sodium hydroxide
and/or acetic acid.
Cold Storage: no
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing
hormone (GnRH or LH-RH). The analog possesses greater potency than the natural hormone. The
chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-
arginyl-N-ethyl-Lprolinamide acetate (salt) with the following structural formula-
Leuprolide acetate is indicated in the palliative treatment of advanced prostatic cancer.
In a clinical study comparing leuprolide acetate injection with DES in patients with metastatic prostate
cancer, adverse reactions were assessed. Common adverse events included hot flashes (55% with
injection vs. 12% with DES), gynecomastia (63% vs. 7%), and decreased libido (11% vs. 7%). Bone
pain occurred in 5% of injection patients compared to 2% with DES. Other reported adverse reactions
included cardiovascular issues like ECG changes/ischemia, gastrointestinal symptoms such as
nausea/vomiting, and urinary tract issues like hematuria. Overall, injection was associated with a
higher incidence of hot flashes and gynecomastia but showed comparable rates of bone pain and other
systemic effects compared to DES in this trial.
Pregnancy: It can cause fetal harm when administered to pregnant women and it should not be used
during pregnancy..
Breastfeeding: It is not known whether leuprolide acetate is excreted in human milk. Because many
drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing
infants, breastfeeding should be discontinued during treatment with leuprolide acetate.
Fertility: Leuprolide acetate may cause reversible suppression of reproductive function. In females,
amenorrhea and suppression of ovarian function have been reported. Regular menstrual cycles may
not resume immediately after discontinuation of therapy. In males, suppression of spermatogenesis
leading to azoospermia or oligospermia has been observed.
Teratogenicity: Leuprolide acetate has the potential to cause fetal harm and is contraindicated during
pregnancy. Women of childbearing potential should use effective contraception during treatment with
leuprolide acetate to prevent potential fetal exposure and related risks.
tell your doctor and pharmacist if you are allergic to leuprolide, goserelin (Zoladex),
histrelin (Supprelin LA, Vantas), nafarelin (Synarel), triptorelin (Triptodur, Trelstar), any
other medications, or any of the ingredients in leuprolide injection. Ask your pharmacist
for a list of the ingredients.
tell your doctor and pharmacist what prescription and nonprescription medications,
vitamins, nutritional supplements, and herbal products you are taking or plan to take.
tell your doctor if you or anyone in your family has or has ever had osteoporosis (condition
where bones are thin and more likely to break); if you have a history of drinking alcohol or
using tobacco products for a long period of time; or if you have or have ever had
depression, seizures, brain tumors, cancer that has spread to the spine (backbone), diabetes,
urinary obstruction (blockage that causes difficulty urinating), blood in your urine, a
prolonged QT interval (a rare heart problem that may cause irregular heartbeat, fainting, or
sudden death), cerebrovascular disease (clogging or weakening of the blood vessels within
the brain or leading to the brain), heart disease,, stroke, hyperlipidemia (elevated
cholesterol)or a low level of potassium, calcium, or magnesium in your blood.
you should know that leuprolide is not to be used in women who are pregnant, can become
pregnant, or are breastfeeding. Tell your doctor if you are pregnant, plan to become
pregnant, or are breast-feeding. Your doctor may perform a pregnancy test to be sure that
you are not pregnant when you begin receiving leuprolide injection. You will need to use a
reliable nonhormonal method of birth control to prevent pregnancy while you are receiving
leuprolide injection. Talk to your doctor about the types of birth control that are right for
you, and continue to use birth control even though you should not have regular menstrual
periods during your treatment. If you think you have become pregnant while receiving
leuprolide injection, call your doctor immediately. Leuprolide injection can harm the fetus.
you should know that leuprolide injection may impair infertility (ability to make babies) in
men
you should know that some patients who receive leuprolide injection may have increasing
difficulty controlling emotions, worsening mood or anxiety, or development of any new
psychiatric illness. Report any new or worsening symptoms to your doctor, nurse, or
pharmacist.