Zovorin Inj
Leucovorin Calcium
Strength: 50MG/5ML
Pack Size: 1 vial
Drug Class: Antidotes, Other; Rescue Agents, Chemotherapy
Dosage and Administration:
Advanced Colorectal Cancer
Either of the following two regimens is recommended:
1- Leucovorin is administered at 200mg/m2 by slow intravenous injection over a minimum of 3 minutes, followed by 5-fluorouracil at 370 mg/m2 by intravenous injection.
2- Leucovorin is administered at 20mg/m2 by intravenous injection followed by 5-fluorouracil at 425 mg/m2 by intravenous injection.
5-Fluorouracil and Leucovorin should be administered separately to avoid the formation of a precipitate.
Treatment is repeated daily for five days. This five-days. This five-day treatment course may be repeated at 4 week (28-day) intervals, for 2 courses and then repeated at 4 to 5 week (28 to 35 day) intervals provided that the patient has completely recovered from the toxic effects of the prior treatment course.
In subsequent treatment course, the dosage of 5-fluorouracil should be adjusted based on patient tolerance of the prior treatment course. The daily dosage of 5-fluorouracil should be reduced by 20% for patients wo experienced moderate hematologic or gastrointestinal toxicity in the prior treatment course, and by 30% for patients who experienced no toxicity in the prior treatment course, 5-fluorouracil dosage may be increased by 10%. Leucovorin dosages are not adjusted for toxicity. Several other doses and schedules of Leucovorin/5-fluorouracil therapy have also been evaluated in patients with advanced colorectal cancer; some of these alternative regimens may also have efficacy in the treatment of this disease. However, further clinical research will be required to confirm the safety and effectiveness of these alternative Leucovorin/5-fluorouracil treatment regimens.
Leucovorin Rescue After High-Dose Methotrexate Therapy
The recommendations for Leucovorin rescue are based on a methotrexate dose of 12 to 15 grams/m2 administered by intravenous infusion over 4 hours (see methotrexate package insert for full prescribing information). 4 Leucovorin rescue at a dose of 15mg (approximately 10mg/m2) every 6 hours for 10 doses starts 24 hours after the beginning of the methotrexate infusion. In the presence of gastrointestinal toxicity, nausea or vomiting, Leucovorin should be administered parenterally. Do not administer Leucovorin intrathecally.
Serum creatinine and methotrexate levels should be determined at least once daily. Leucovorin administration, hydration, and urinary alkalization (pH of 7.0 or greater) should be continued until the methotrexate level is below 5 X 10-8 M (0.05 micromolar). The Leucovorin dose should be adjusted or Leucovorin rescue extended based on the following guidelines:
| Guidelines for Leucovorin dosage and administration do not administer Leucovorin intrathecally | ||
| Clinical Situation | Laboratory Findings | Leucovorin Dosage Dosage and Duration |
| Normal Methotrexate Elimination | Serum methotrexate level approximately 10 micromo-molar at 24 hours after administration. 1 micromolar at 72 hours. | 15mg PO, IM, or IV q 6 hours for 60 hours (10 doses starting at 24 hours after start of methotrexate infusion). |
| Delayed late Methotrexate Elimination | Serum methotrexate level remaining above 0.2 micromolar at 72 hours, and more than 0.05 micromolar 96 hours after administration. | Continue 15 mg PO, IM or IV q 6 hours, until methotrexate level is less than 0.05 micromolar. |
| Delayed Early Methotrexate Elimination and/or Evidence of Acute Renal Injury | Serum methotrexate level of 50 micromolar or more at 24 bours after administration, OR; a 100% or greater increase in serum creatinine level at 24 hours after methotrexate administration (e.g. an increase from 0.5 mg/dLto a level of 1 mg/dL or more). | 150 mg IV q 3 hours, until methotrexate level is less than 1 micromolar; then 15mg IV q 3 hours until methotrexate level is less than 0.05 micromolar. |
Patients who experience delayed early methotrexate elimination are likely to develop reversible renal failure. In addition to appropriate Leucovorin therapy, these patients require continuing hydration and urinary alkalization, and close monitoring of fluid and electrolyte status, until the serum methotrexate level has fallen to below 0.05 micromolar and the renal failure has resolved.
Some patients will have abnormalities in methotrexate elimination or renal function following methotrexate administration, which are significant but less severe than abnormalities described in the table above. These abnormalities may or may not be associated with significant clinical toxicity. If significant clinical toxicity is observed, Leucovorin rescue should be extended for an additional 24 hours (total of 14 doses over 84 hours) in subsequent courses of therapy. The possibility that the patient is taking other medications which may interfere with methotrexate elimination or binding ot serum albumin) should always be reconsidered when laboratory abnormalities or clinical toxicities are observed.
Impaired Methotrexate Elimination or Inadvertent Overdosage
Leucovorin rescue should begin as soon as possible after an inadvertent overdosage and within 24 hours of methotrexate administration when there is a delayed excretion (see WARNINGS section). Leucovorin 10 mg/m2 should be administered IM, IV, or PO every 6hours until the serum methotrexate level is less than 10-8 M. In the presence of gastrointestinal toxicity, nausea, or vomiting, Leucovorin should be administered parenterally. Do not administer Leucovorin intrathecally.
Serum creatinine and methotrexate levels should be determined at 24 hour intervals. If the 24 hour serum creatinine has increased 50% over baseline or if the 24 hour methotrexate level is greater than 5 X 10-6 M or the 48 hour level is greater than 9 X 10-7 M, the dose of Leucovorin should be increased to 100mg/m2 IV every 3 hours until the methotrexate level is less than 10-8 M.
Hydration (3 L/d) and urinary alkalization with sodium bicarbonate solution should be employed concomitantly. The bicarbonate dose should be adjusted to maintain the urine pH at 7.0 or greater.
Megaloblastic Anemia Due to Folic Acid Deficiency
Up to 1 mg daily. There is no evidence that doses greater than 1 mg/day have greater efficacy than those of 1 mg; additionally, loss of folate in urine becomes roughly logarithmic as the amount administered exceeds 1 mg.
Because of the calcium content of the Leucovorin solution, no more than 160mg of Leucovorin should be injected intravenously per minute (16 mL of a 10mg/mL, or 8 mL of a 20 mg/mL solution per minute).
Parenteral products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Cold Storage: no
Leucovorin is one of several active, chemically reduced derivatives of folic acid. It is useful as an antidote to drugs which act as folic acid antagonists.
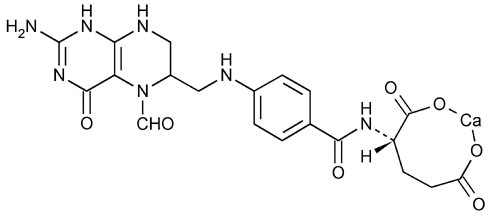
Also known as folinic acid, Citrovorum factor, or 5-formyl-5,6,7,8-tetrahydrofolic acid, this compound has the chemical designation of Calcium N-[p-[[[(6RS)-2-amino-5-formyl 5,6,7,8-tetrahydo-4-hydroxy-6 pteridinyl]methyl]amino]benzoyl]-L-glutamate (1:1). The structural formula of Leucovorin calcium is :
Leucovorin calcium rescue is indicated after high dose methotrexate therapy in osteosarcoma. Leucovorin calcium is also indicated to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent overdosages of folic acid antagonists.
Leucovorin calcium is indicated in the treatment of megaloblasticanemias due to folic acid deficiency when oral therapy is not feasible.
Leucovorin calcium is indicated for use in combination with 5-fluorouracil to prolong survival in the palliative treatment of patientswith advanced colorectal cancer. Leucovorin should not be mixed in the same infusion as 5-fluorouracil because a precipitate may form.
Leucovorin calcium is indicated in the treatment of megaloblastic anemias due to folic acid deficiency when oral therapy is not feasible. Leucovorin is also indicated for use in combination with 5-fluorouracil to prolong survival in the palliative treatment of patients with advanced colorectal cancer.
- Read the label carefully before use
- Keep out of the reach of children
- General Parenteral administration is preferable to oral dosing if there is a possibility that the patient may vomit and not absorb the Leucovorin. Leucovorin has no effect on non-hematologic toxicities of methotrexate such as the nephrotoxicity resulting from drug and/or metabolite precipitation in the kidney. Since Leucovorin enhances the toxicity of fluorouracil, Leucovorin/5-fluorouracil combination therapy for advanced colorectal cancer should be administered under the supervision of physician experienced in the use of antimetabolite cancer chemotherapy. Particular care should be takenin the treatment of elderly or debilitate colorectal cancer patients, as these patients may be at increased risk of severe toxicity.
- Laboratory Tests Patients being treated with the Leucovorin/5-fluorouracil combination should have a CBC with differential and platelets prior to each treatment. During the first two courses a CBC with differential and platelets has to be repeated weekly and thereafter once each cycle at the time of anticipated WBC nadir. Electrolytes and liver function tests should be performed prior to each treatment for the first three cycles than prior to every other cycle. Dosage modifications of fluorouracil should be instituted as follows, based on the most severe toxicities.
Diarrhea and/or Stomatitis WBC/mm3 Nadir Platelets/mm3 Nadir 5-FU dose Moderate 1,000-1,900 25-75,000 Decrease 20% Severe <1,000 <25,000 Decrease 30% If no toxicity occurs, the 5-fluorouracil dose may increase 10%. Treatment should be deferred until WBCs ARE 4,000/mm3 and platelets 130,000/mm3. If blood counts do not reach these levels within two weeks, treatment should be discontinued. Patients should be radiological examination as needed. Treatment should be discontinued when there is clear evidence of tumor progression.