Zuvitere
Docetaxel
Strength: 20mg / 80mg / 120mg
Pack Size: 1 vial
Drug Class: Taxane cytotoxic.
Dosage and Administration:
For treatment of patients with locally advanced or metastatic carcinoma of the breast after progression during anthracycline-based therapy for metastatic disease or relapse during anthracycline-based adjuvant therapy, the recommended dose of ZUVITERE is 60-100 mg/m2 administered intravenously over one hour every three weeks.
duringanthracycline-based adjuvant therapy, the recommended dose of ZUVITERE is 60-100 mg/m2 administered intravenously over one hour every three weeks.
For treatment of patients with advanced or metastatic non-small cell lung cancer after the failure of prior platinum containing therapy , the recommended dose of docetaxel is 75-100 mg/m2 I.V. over one hour every 3 weeks.
Premedication Regimen : All patients should be premedicated with oral corticosteroids such as
Dexamethasone 16 mg. per day (e.g. 8 mg. BD for 5 days starting 1 day prior to ZUVITERE administration in order to reduce the incidence and severity of fluid retention as well as the severity of hypersensitivity reaction.
Dosage Adjustments During Treatment : Patients who are dosed initially at 100 mg/m2 and who experience either febrile neutropenia neutrophils < 500 cells/mm3, severe or cumulative cutaneous reactions, or severe peripheral neuropathy during therapy should have the dosage adjusted from 100 mg/m2 to 75 mg/m2. If the patient continues to experience these reactions the dosage should either to decreased from 75 mg/m2 to 55 mg/m2 or the treatment should be discontinued, Conversely, patients who are dosed initially at 60 mg/m2 and do not experience febrile neutropenia,neutrophils< 500 /mm2 for more than one week, severe or cummulative cutaneous reactions or severe peripheral neuropathy during docetaxel therapy may tolerate higher doses.
Preparation and Reconstitution of Injection:
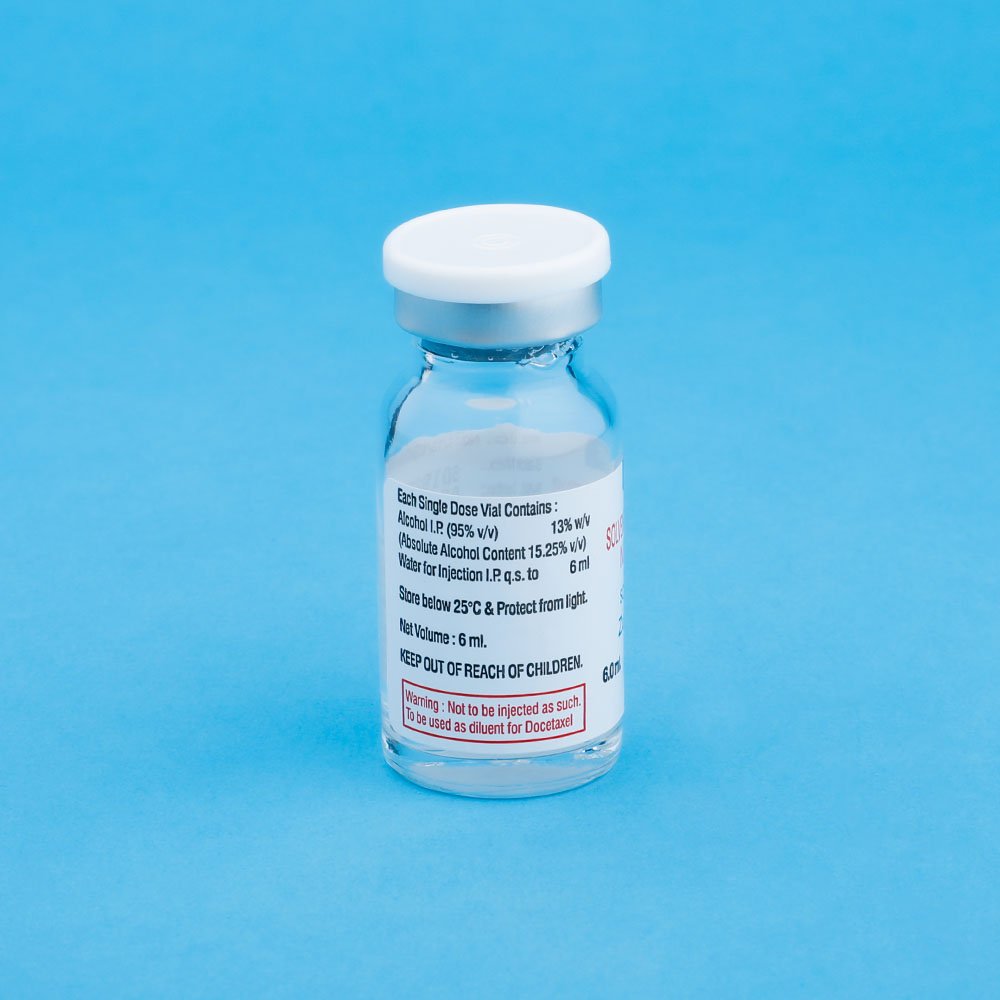
Preparation of the Premix solution: Docetaxel for Injection Concentrate require dilution prior to use. A Sterile, non-pyrogenic, single dose diluent containing 13% w/v alchol IP in water for Injection is supplied for that purpose, Remove the appropriate number of vials of docetaxel Injection Concentrate from the refrigerator. Allow the vials to stand at room temperature for approximately 5 minutes. Using a syringe fitted with a needle, aseptically withdraw the entire contents of the solvent for Docetaxel Injections concentrate vial and gently transfer it to the corresponding vial of Docetaxel Injection Concentrate (i.e. 9.9 ml +5% for ZUVITERE – 120 ; 7.33 ml, + 5% for ZUVITERE – 80, 1:83 ml. + 5% ZUVITERE – 20) This will assure a minimal extractable volume of 12 ml. (for ZUVITERE-120) or 8 ml. (for ZUVITERE – 80) or 2 ml. (for ZUVITERE -20) of the premix solution containing 10 mg. Docetaxel / ml. for example, a patient requiring a dose of 140 mg. Docetaxel would require 14 ml. premix solution.
Preparation of the infusion solution: Asceptically withdraw the required amount of ZUVITERE premix solution (10 mg. docetaxel /ml) with a calibrated syringe and inject the required volume of premix solution into a 150 ml. Infusion bag or bottle of either sodium chloride intravenous infusion IP or Dextrose Intravenous infusion IP to produce a final concentration of 0.9 mg/ml.
1) If dose greater than 240 mg. of Docetaxel is required use a large volume of the infusion vehicle so that a concentration of 0.9 mg /ml Docetaxel is not exceeded.
2) Thoroughly mix the infusion by manual rotation.
3) As with all products ZUVITERE should be inspected visually for particulate matter or discoloration prior to administration whenever the solution and container permit. If ZUVITERE for Injection premix solution or infusion solution is not clear or appears to have precipition, the solution should be discarded.
ZUVITERE : Infusion solution should be administered intravenously as a one-hour infusion under ambient room temperature and light conditions contact of the undiluted cncentrate with plasticized PVC equipment or devices used to prepare solutions for infusion is not recommended. In order to minimise patient exposure to the plasticizer DEHP (di-2-ethylhexyl phthalate), which may be leached from PVC infusion bags or sets, diluted ZUVITERE solution should be stored in bottle (glass, polypropylene) or plastic bags (polypropylene, polyolefin) and administered through polyethyle-lined administration.
disease has not been established. )
Cold Storage: yes
Docetaxel is an antineoplastic agent belonging to the taxoid family. It is prepared by semisynthesis beginning with a precursor extracted from the renewable needle biomass of yew plants. The Chemical name for Docetaxel is (2R, 3S)-N-Carboxy-3-Phenylisoserine, N-tert-butylester. 13 ester with 5b-20-epoxy-1,2a,4,7b, 13a-hexahydroxytax-11-en-9-one 4-acetate 2- benzoate, trihydrate.
Docetaxel is a white to almost white powder with an empirical formula of C43H53NO1413H2O2 and a molecular weight of 861.9 It is highly lipophilic and practically insoluble in water.
Docetaxel has the following structural formula:
ZUVITERE for Injection concentrate is indicated for the treatment of patients with locally advanced or metastatic breast cancer, who have progressed during anthracycline based therapy or have relapsed
duringanthracycline based adjuvant therapy. It is also indicated for the treatment of advanced or metastatic non-small cell lung cancer affect failure of platinum containing chemotherapy.
Docetaxel has been shown to be efficacious as a monotherapy in both chemo-naïve and resistant NSCLC8,9,10 and is approved on a 3-week schedule at a dose of 75 mg/m2.
General: Responding patients may not experience an improvement in performance status on therapy and may experience worsening. The relationship between changes in performance status, response to therapy and treatment – related side effects has been established.
Hemotologic Effects: In order to monitor the occurrence of myelotoxicity, it is recommended that frequent peripheral blood cell counts be performed on all patients receiving ZUVITERE patients should not be retreated with subsequent cycles of ZUVITERE, until neutrophils recover to a level> 1,500 cells.mm3 and platelets recover to a level > 100,000 cells/mm2
A 25% reduction in the dose of docetaxel is recommended during subsequent cycle following severe neutropenia (<500 cells/mm3) lasting 7 days or more, febrile neutropenia, or grade 4 infections in a docetaxel cycle.
Hypersensitivity Reactions: Hypersensitivity reactions may occur within a few minutes following initiation of an ZUVITERE infusion. If minor reaction such as flushing or localized skin reactions occur, of ZUVITERE and aggressive therapy. All patients shouldbepremedicated with an oral corticosteroid prior to the initiation of the infusion of ZUVITERE
Cutaneous: Localized erythoma of the extremities with edema followed by desquamation has been observed. In case of severe skin toxicity, an adjustment in dosage is recommended (See Dosage and Administration section) Fluid REtention: Severe fluid retenation has been reported following docetaxel therapy. Patients should be premedicated with oral corticosteroids prior to each administration to reduce the incidence and severity of fluid retention (see Dosage and Administration section). Patients with pre-existing effusions should be closedly monitored from the first dose for the possible exacerbation of the effusions. In patients who received the recommended premedication, moderate fluid retention was completely, but sometimes slowly reversible following discontinuation of docetaxel (median of 29 weeks). The median cumulative dose to onset of moderate or severe fluid retention was 7052 mg/m2 in patients receiving premedication. Patients developing peripheral edema may be treated with standard measure, e.g., salt restriction, oral diuretic (S)
Neurologic : Severe neurosensory symptoms (paresthesia, dysesthesia, pain) were observed among 7% of 134 patients with anthracycline-resistant breast cancer. When these occur, dosage must be adjusted. if symptoms persist, treatment should be discontinued (see Dosage Administration section). Patients who experienced neurotoxicity in clinical trials and for whom follow-up information on the complete resolution of the event was available has spontaneous reversal of symptoms within a median of 9 weeks from onset (range : 0 to 106 weeks) and only about 3.8% of patients required discontinuation due to neurotoxicity. Peripheral motor neuropathy mainly manifested as distal extremity weakness occured in 13.4% ( 7.1% severe) of the 127 anthracycline-resistnat breast cancer patients with normal LFTs. No neuromotor toxicity was reported in the 7 patients with elevated LFTs.
Extremity weakness occured in 13.4% (7.1% severe) of the 127 anthracycline-resistant breast cancer patients with normal LFTs. No neuromotor toxicity was reported in the 7 patients with elevated LFTs.
Asthenia: Severe asthenia has been reported in 111% of the patients but has led to treatment discontinuation in only 2.6% of the patients. Severe asthenia was reported in 23% of 134 patients with anthracycline-resistant breast cancer and 5.5% of the 786 cycles received. Symptoms of fatigue and weakness may last a few days up to several weeks and may be associated with deterioration of performance status in patients with progressive disease.
Call your doctor right away if you have a rash, itching, fever or chills, trouble breathing or swallowing, a fast or irregular heartbeat, or any swelling of your hands, face, mouth, or throat with this medicine. Docetaxel may cause edema or fluid retention, which means your body is keeping too much water.